Commercializing a Major Medical Discovery
BioAegis is pursuing a game-changing approach to address medical issues that have previously been seen as out of reach — that is, to prevent the damage caused by excess inflammation but to do this without suppressing the immune system, without putting patients at risk for infection.
“We are simply capitalizing on Evolutionary Wisdom with a natural protein, plasma gelsolin, which has fulfilled this role for vertebrates for millennia.” – Susan L. Levinson, CEO
A Pipeline Intent on Validating Efficacy for Multiple Indications
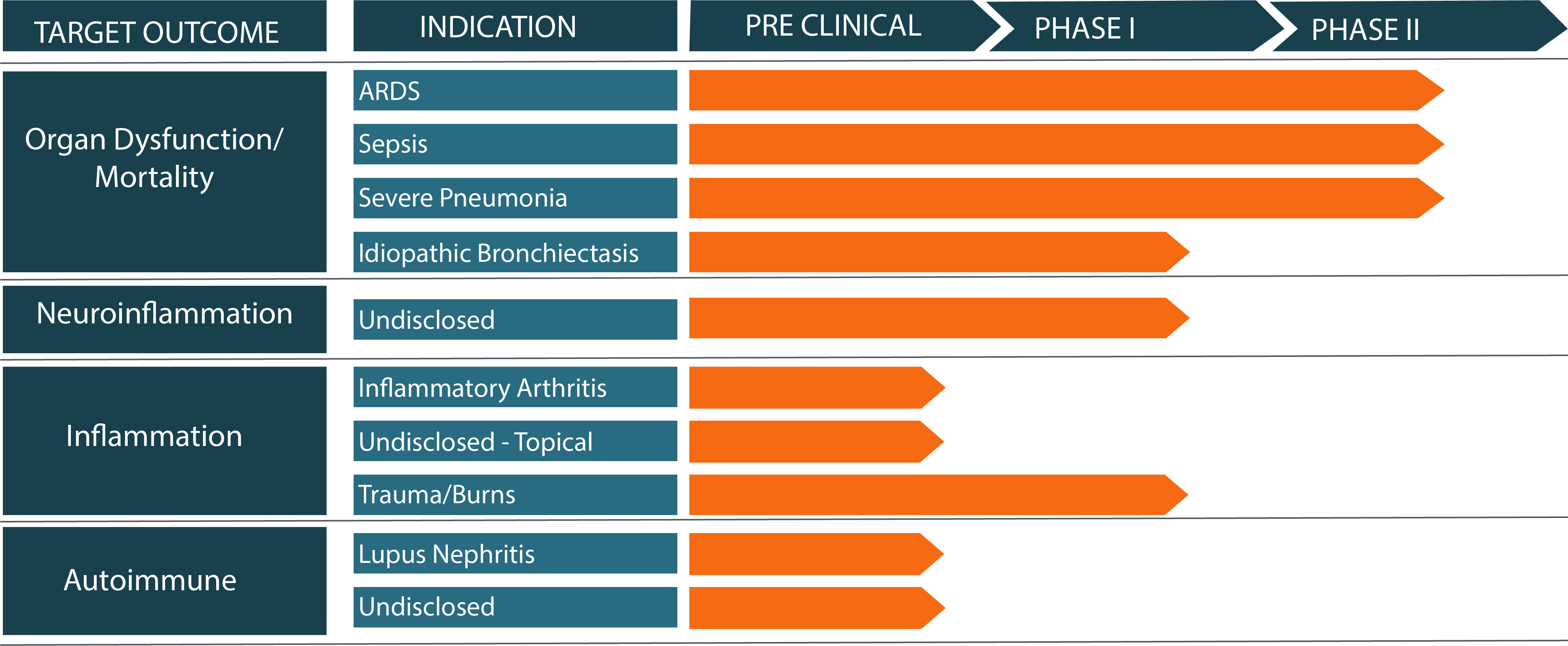
Current Focus: Acute Respiratory Distress Syndrome (ARDS)
ARDS has severe consequences for patients with NO current therapies to treat the condition other than supportive care. In the US alone, ARDS affects over 700,000 patients per year or roughly 10% of all ICU admissions. The mortality rate for ARDS is ~40%. This mortality as well as damage to organs such as the lung is driven by excess inflammation.
Plasma gelsolin, a normal human protein which regulates the inflammatory process, is critically depleted in ARDS patients and has been shown to address both injurious and infectious inflammation in multiple studies. Treating patients with recombinant human plasma gelsolin (rhu-pGSN) holds much promise for addressing the over exuberant inflammatory response that can lead to ARDS and the subsequent high mortality levels. A Phase 2 clinical trial of rhu-pGSN to treat critical care ARDS patients is planned for 2023.
We are collaborating with the BARDA DRIVe Solving Sepsis program, to further develop plasma gelsolin for ARDS. BARDA is a division of the US National Institutes of Health and ASPR (Administration for Strategic Preparedness and Response).
WHY ARDS IS OUR CURRENT TARGET
Depletion of Plasma Gelsolin Leads to Dysregulated Inflammation
$50B PIPELINE MARKET OPPORTUNITY
As a master regulator of the immune system, rhu-pGSN is a game-changing anti-inflammatory without immunosuppressive properties. In a wide range of diseases, it balances the inflammatory process to prevent the spread of excess inflammation while simultaneously enhancing antimicrobial defense; thus preventing severe consequences, and greatly improving survival.
We are pursuing multiple secondary indications with unique formulations suited to chronic therapy. Data from animal studies support efficacious treatment via routes of administration suitable for patient-delivered therapy, including by inhalation and subcutaneous injection.
Applying Cutting Edge Manufacturing
We have partnered with a major contract manufacturer to produce GMP material for clinical and commercial use. Highly productive and efficient processes have been developed for manufacture of this recombinant protein.
Multiple GMP manufacturing runs have been completed and drug product suitable for hospital use has been produced. Long-term drug product stability exceeds 72 months and currently supports a 48 month shelf life.
