Plasma Gelsolin's Biological Role:
Critical Regulator of Inflammation
Plasma gelsolin (pGSN) is a naturally occurring blood protein that is abundant in healthy individuals. Its primary role is to keep inflammation localized to the site of injury and enhance the function of the innate immune system, the first line of defense against pathogen threats.
Disease-driven inflammation consumes pGSN levels in the blood. When pGSN levels drop, the body loses an important control system. Inflammation can spread instead of staying contained, causing tissue and organ damage. The immune system also becomes less effective at fighting infection. Plasma gelsolin deficiency (PGD) is a strong biomarker of poor outcomes across numerous acute and chronic inflammatory conditions.
Multifaceted Mechanism that Addresses the Complexity of Inflammation
Plasma gelsolin’s non-immunosuppressive mechanism enables the host to mount a pathogen-agnostic response to infection while also avoiding the dysfunction of uncontrolled inflammation. In multiple animal disease models, administration of rhu-pGSN improves survival, reduces morbidity and protects critical organs.
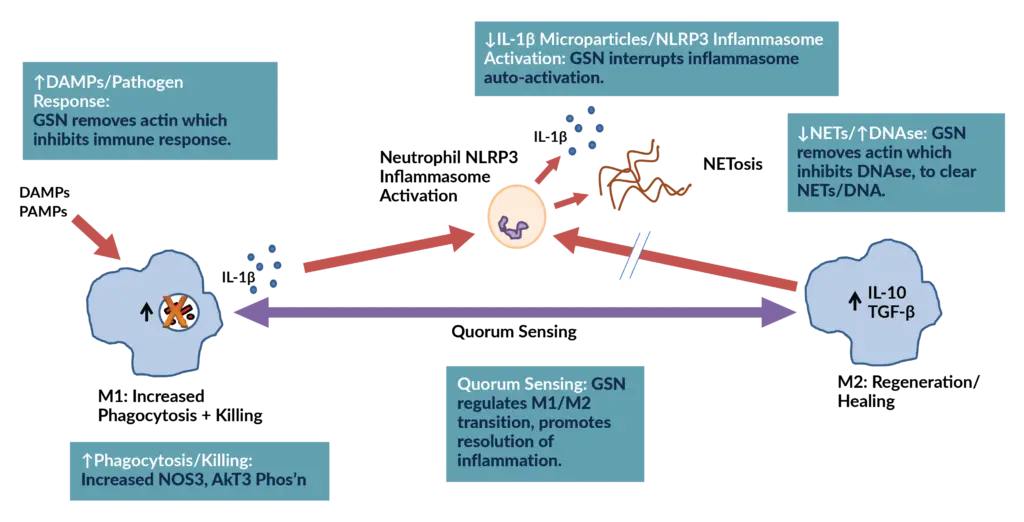
Explore the 4 mechanisms behind pGSN's unique role in immune regulation.
1: Modulates the activation of the NLRP3 inflammasome and minimizes the production of IL-1ß and inflammatory microparticles.
pGSN interrupts NLRP3 inflammasome activation, a key driver of the body’s inflammatory cascade. When this pathway becomes activated, it triggers excess release of IL-1β and other potent cytokines, leading to tissue injury, systemic inflammation, and worsening disease severity.
Clinical Benefit:
- Counteracts the destructive inflammation driven by IL-1β and the NLRP3 inflammasome.
- Mitigates microparticle-driven inflammation, which contributes to endothelial injury, coagulopathy, and organ dysfunction.
2: Enhances Uptake and Killing of Microbial Pathogens by Innate Immune Cells
pGSN directly interacts with the white blood cells (macrophages), to enhance pathogen recognition by scavenger receptors and to activate pathogen killing via NOS3 phosporylation.
Clinical Benefit:
- Enhances pathogen defense against multiple gram-positive and gram-negative organisms, including antibiotic resistant strains.
3: Binds to and Removes Harmful Inflammatory Mediators and Toxic Actin Released from Cells.
pGSN binds to a broad range of inflammatory mediators such as LPA, LPS, Sphingosine-1-phosphate, Amyloid-β, PAF, PIP2, actin, and inflammatory microparticles.
pGSN clears toxic actin released from damaged cells, which is known to drive inflammation, promote immune dysfunction, and impair host defense. By dismantling toxic actin networks, pGSN also helps break down biofilms and reduces cell-free DNA/NETs that contribute to persistent inflammation and infection.
Clinical Benefit:
- Restores effective host defense by counteracting the immunosuppressive effects of toxic actin and biofilms.
- Prevents systemic spillover of inflammatory mediators, helping protect organs from collateral damage.
4: Regulates Macrophage Shift from Inflammatory (M1) to Healing (M2) Phenotype
Quorum-sensing, a property associated with cell density and well-known in bacteria for decades has now been described in macrophages where cell density triggers a switch from an inflammatory (M1) to an anti-inflammatory (M2) phenotype.
pGSN was shown to be the temporal regulator for this process, finetuning the inflammatory response to limit the risk of a dysfunctional hyperinflammatory state.
Clinical Benefit:
- Promotes timely transition to healing, supporting tissue repair and recovery.
- Reduces risk of hyperinflammatory injury, which can drive organ dysfunction in severe disease.
There are many layers to the science underlying gelsolin's role as a master regulator in immunity. The more we learn about this key signaling protein, the more we look forward to bringing rhu-pGSN to patients.
Susan L. Levinson, PhD, CEO of BioAegis
Efficacy and Broad Therapeutic Potential:
Validated in > 20 Clinically Relevant Animal Models
Individual labs demonstrate gelsolin’s efficacy in diseases where current competitive therapies fail to deliver patient benefit.
Inflammation/CV/Metabolic
- Ischemic Stroke Model
- Inflammatory Bowel Disease
- Diabetes Type 2: Multiple Models
- Pain: Central Periheral Models
Injury/Trauma
- Burns – Lung Microvascular Permeability
- Radiation Exposure
- Hyperoxia Acute Lung Injury
Infection
- Enhanced Microbial Uptake and Killing in Human Alveolar Macrophages
- Gram Positive/Gram Negative
- Influenza
- Infectious Peritoniti
Neurological
- Alzheimer’s Disease
- Multiple Sclerosis (EAE)
- Neuroinflammation
- Decompression sickness
- CO poisoning
Collaborating With Leading Institutions Worldwide
BioAegis has worked with distinguished institutions worldwide. These collaborations have enhanced our ability to recognize additional commercial opportunities, extend the research effort that is ongoing in our own laboratory, and refine and drive current programs forward.
